Medical Device Packaging Trays
Custom thermoformed trays engineered for device protection and procedural efficiency
Janco designs and manufactures medical device trays that support sterile workflows, consistent instrument layout, and protection during transport, storage, and clinical use. All trays are produced in ISO 13485 certified facilities, manufactured in Class 8 cleanrooms, and packaged in Class 7 environments.
With more than 60 years of experience, Janco develops custom tray systems for surgical, sterile processing, diagnostic, and specialty applications, guiding customers through design, prototyping, and production.

Our Medical Tray Capabilities
Janco designs fully custom medical device trays built for accuracy, sterile handling, and dependable procedural use. Each tray is engineered for precise device fit, structured instrument presentation, and compatibility with sterile processing workflows. Capabilities include:
- Custom thermoformed trays for single-use or reusable systems
- Compression-molded trays for rigid, durable structures and protected instrument retention
- Multi-compartment configurations for implants, sutures, packaged components, and procedural sequencing
- Lids, peel tops, locking features, and engineered dividers that support clean handling and reduce intra procedure search time
- ISO 14644-1 Class 8 cleanroom production with Class 7 cleanroom packaging to control airborne particulates
- Material guidance for durability, biocompatibility, and sterilization compatibility
- Prototyping, design refinement, and validation testing to ASTM, ISO, or customer standards, with full traceability back to material lot numbers
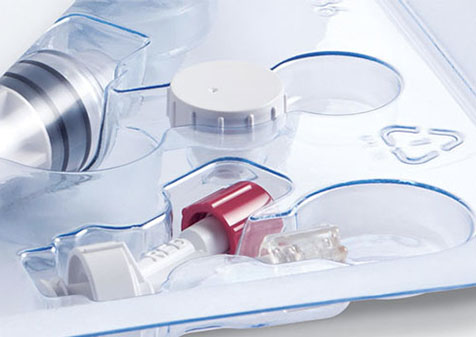
Types of Medical Device Packaging Trays
Janco manufactures custom tray formats designed to support specific procedural, sterilization, and handling requirements. Core tray types include:
- Procedure trays engineered to sequence instruments in chronological order for efficient surgical workflow
- Sterilization trays designed to secure and protect instruments through cleaning, sterilization, and storage, using materials compatible with multiple sterilization methods
- Single-use disposable trays that provide sterile-ready, cost-effective packaging without reprocessing demands
- Multi-compartment trays with rigid thermoformed sections that isolate implants, sutures, and packaged components for fast, error-reducing access
- Extended-length specialty trays available up to 70 inches for cardiovascular and other procedures requiring long instruments
Applications and Industries Served
Janco’s medical device trays are used across clinical environments that require reliable instrument organization, sterile handling, and durable protective packaging. Our tray systems support applications such as implant delivery, procedural sequencing, diagnostic workflows, and sterile processing across:
- Orthopedics
- Vascular
- Respiratory
- Endoscopy
- Laboratory
- Cardiology
- Ophthalmic
- Spinal and Biologics
- Dental
- Pharmaceutical
- Veterinary
- Drug delivery
Why Janco
Medical manufacturers choose Janco for tray systems that must perform reliably in sterile, regulated, and high-stakes clinical environments. With more than 60 years of specialization in medical packaging, we provide:
- ISO 13485 certification and FDA registration for documented quality and regulatory alignment
- Class 7 and Class 8 cleanroom manufacturing and packaging to protect against airborne contamination
- Advanced thermoforming and compression molding for precise geometries and consistent part performance
- Material expertise that ensures durability, biocompatibility, and sterilization compatibility
- Regulatory support including ASTM, ISO, and FDA validation testing to help streamline approvals
- Engineering collaboration that reduces design risk, improves manufacturability, and supports predictable lead times
- Proven production stability built on decades of medical-industry experience and long-term customer partnerships
Ready to begin your medical device tray project?
Contact Janco Medical to request a quote or discuss custom thermoformed and molded tray solutions for your device, procedural kit, or sterile packaging system.
Capabilities Form
"*" indicates required fields